2025 Wisconsin Collaborative Clinical Research Professionals Conference
Schedule
Thu Apr 17 2025 at 09:00 am to 04:30 pm
UTC-05:00Location
Goodman Community Center | Madison, WI

About this Event
Join others who have a shared interest in advancing clinical research and improving health outcomes in our state and beyond! The conference will be held April 17, 2025 at the Goodman Community Center in Madison. The event will bring together the “Voices of Clinical Research,” participants, researchers, industry partners, academic health centers, students, and community organizations who collectively work to advance clinical research. This will be a terrific opportunity to engage with fellow stakeholders, build partnerships, and exchange ideas on research participation, diversity, technology, and career development.
Please Note: When registering, please select ONE General Admission ticket and ONLY ONE Breakout Session (listed below) ticket. The breakouts will occur simultaneously.
Breakout Sessions:
Beyond Schedule Surfing: Leveraging the Electronic Health Record for Research Recruitment - In this breakout session, Carla Croft, RN Manager for Health Link Clinical Research at UW Health, will provide an overview of how to use the electronic health record (EHR) to identify patients who are potentially eligible for a research study. It will review the features of an electronic health record that supports research recruitment and present a framework to translate research protocol eligibility criteria into recruitment strategy in the electronic health record. This will include an overview of strategies and tools available within Epic at UW Health (also known as Health Link).
Beyond the Checklist: Strategies for Navigating FDA Audits in Clinical Research - Adarsh Sukhwal, Director of Clinical Trials Implementation & Coordination at the Clinical Trial Institute at UW-Madison, will provide an overview of strategies for navigating FDA audits in clinical research, including key dos and don’ts for ensuring a smooth inspection process. The discussion will cover preparation tips for your team, documentation management, and effective communication with FDA inspectors. Emphasis will be placed on how to prepare for an FDA audit both during the conduct of research and in advance of a scheduled review. Throughout the session, practical insights and strategies will be shared to help ensure audit readiness and regulatory compliance, leading to a successful FDA inspection experience.
Research Recruitment for Marginalized Populations: Rebuilding Trust & Strengthening Communities- In this interactive breakout session, Nandi Nandihalli, MS, LPC, will explore innovative strategies for engaging and recruiting marginalized populations in clinical research. Marginalized communities, including racial and ethnic minorities, LGBTQ+ individuals, and socioeconomically disadvantaged groups, have historically been underrepresented in research due to systemic barriers, mistrust, and lack of culturally responsive approaches. This session will address these challenges by focusing on rebuilding trust, fostering authentic community partnerships, and implementing inclusive recruitment practices.
Parking Information:
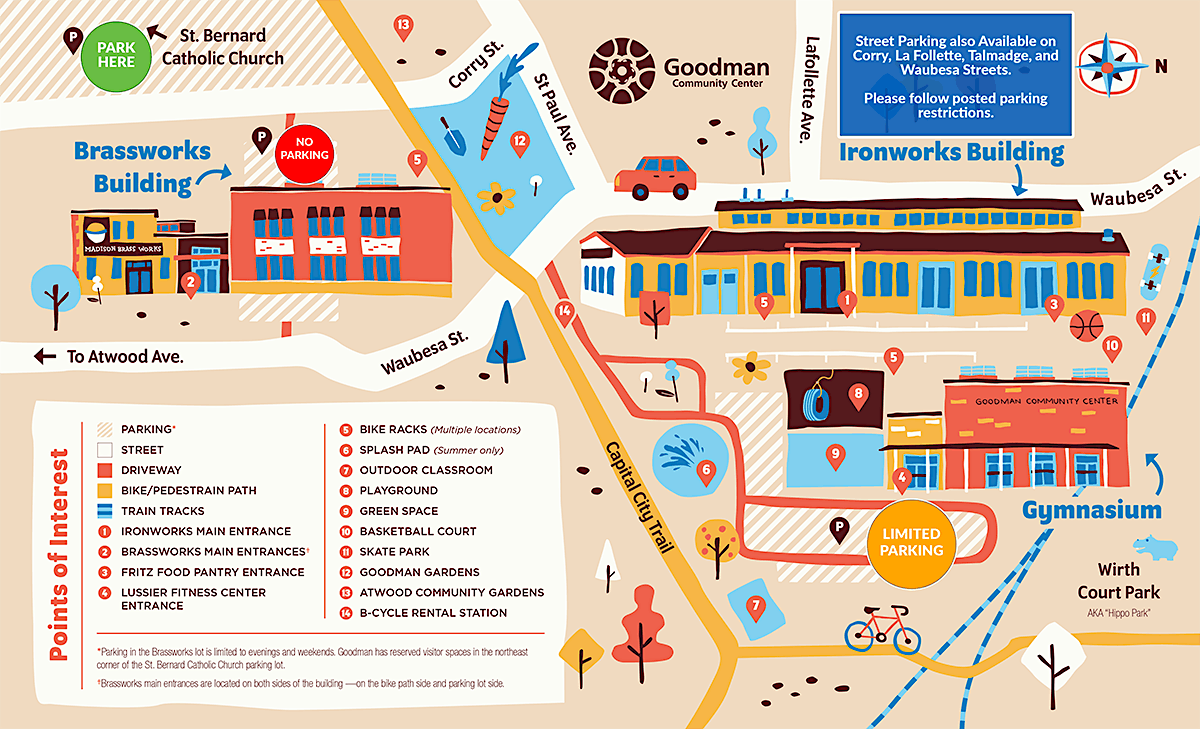
Please note that due to some ongoing construction affecting the St. Bernard's Church parking lot, parking options are somewhat limited this year. We ask that you arrive early to maximize your parking options. We apologize in advance for any inconvenience due to parking restrictions.
St. Bernard’s Church (2450 Atwood Avenue) parking lot: Goodman has reserved visitor spaces in the northeast corner of the church parking lot. Please only park in the YELLOW stalls.
Goodman Ironworks Building (214 Waubesa Street): Open space in this lot is limited.
Street parking: Free parking is available on the following streets around the Center: Corry, LaFollette, St. Paul, Talmadge, and Waubesa. However, please note posted parking restrictions on these streets.
Marquette Street: For those who are able, Marquette Street is a short walk from the Ironworks building via the Capital City Trail walking/biking path.
Please Note: The Brassworks Building (206 Waubesa Street) parking lot WILL NOT be available for parking during the conference.
If you need an accommodation for this event, please contact at least two weeks in advance.
Agenda
🕑: 09:00 AM - 09:30 AM
Continental Breakfast
Info: Sponsored by the UW–Madison M.S. in Biotechnology Program.
🕑: 09:30 AM - 09:40 AM
Welcome
🕑: 09:40 AM - 10:40 AM
Large Group Keynote Session
Host: Dr. Lee Ann Conard
🕑: 10:40 AM - 11:00 AM
Interactive Session with Dr. Lee Ann Conard
🕑: 11:00 AM - 11:15 AM
Break & Vendor Time
🕑: 11:15 AM - 12:30 PM
Panel Discussion: Why We Trial
Info: Participants from 3 different clinical trials share their stories and experiences.
🕑: 12:30 PM - 01:30 PM
Lunch (Provided)
Info: Sponsored by The William L. Weston Foundation.
🕑: 01:30 PM - 02:40 PM
Breakout Sessions (3 options)
Info: FDA Audits; Leveraging the Electronic Health Record for Research Recruitment; or Research Recruitment for Marginalized Populations
🕑: 02:40 PM - 03:00 PM
Break
🕑: 03:00 PM - 04:00 PM
Large Group Session - OrganOx Case Study
Host: Lynn Berg
Info: Learn about the clinical trial journey of OrganOx's metra, an FDA-approved medical device that helps improve liver transplant outcomes.
🕑: 04:00 PM - 04:30 PM
Q&A and Closing Remarks
Where is it happening?
Goodman Community Center, 214 Waubesa Street, Madison, United StatesEvent Location & Nearby Stays:
USD 0.00




















